Sandoz receives approval by European Commission for Hyrimoz® (adalimumab) high-concentration formulation
The Pharma Data
APRIL 4, 2023
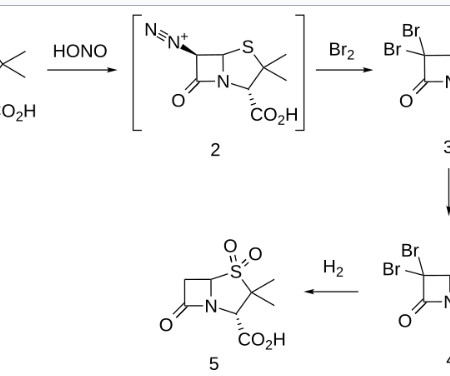
As part of the comprehensive submission package to the European Marketing Authorization, Sandoz conducted a Phase I pharmacokinetics (PK) bridging study comparing its approved adalimumab 50 mg/mL 2 with the 100 mg/mL (HCF).









Let's personalize your content