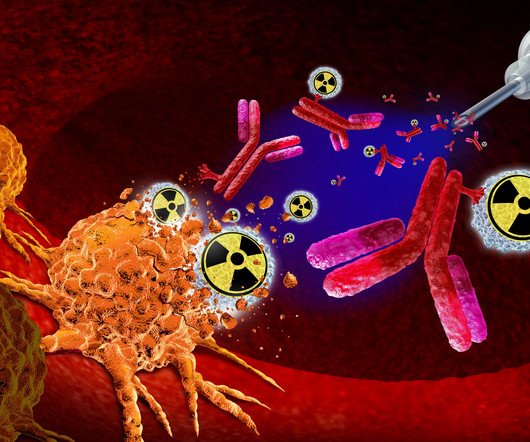
Why radiopharmaceuticals are gaining ground in the fight against cancer
Drug Target Review
JUNE 6, 2025
At the forefront of this transformation is Dr Ebrahim Delpassand, a nuclear medicine physician and the driving force behind RadioMedix (RMX), a radiopharmaceutical company focused on developing targeted diagnostics and therapies. In 2006, he founded RadioMedix, a clinical stage biotechnology company, based in Houston, Texas.








































Let's personalize your content