Gepirone
New Drug Approvals
JUNE 11, 2025
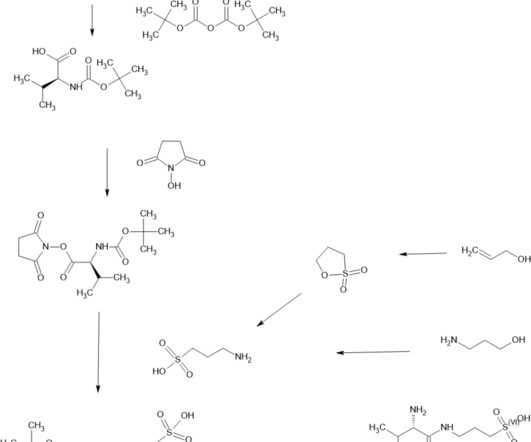
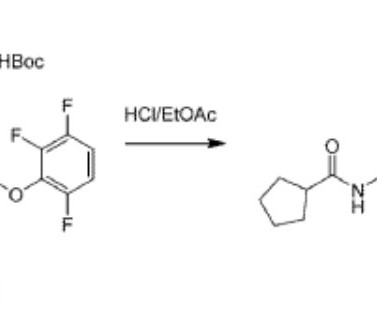
5] History Gepirone was developed by Bristol-Myers Squibb in 1986, [5] but was out-licensed to Fabre-Kramer in 1993. Archived from the original on 24 September 2017. 1] It is taken orally. [1] 1] Side effects of gepirone include dizziness , nausea , insomnia , abdominal pain , and dyspepsia (indigestion). [1] 17 March 2016.



















Let's personalize your content