Advancements in hit identification for membrane protein drug discovery
Drug Target Review
APRIL 7, 2025
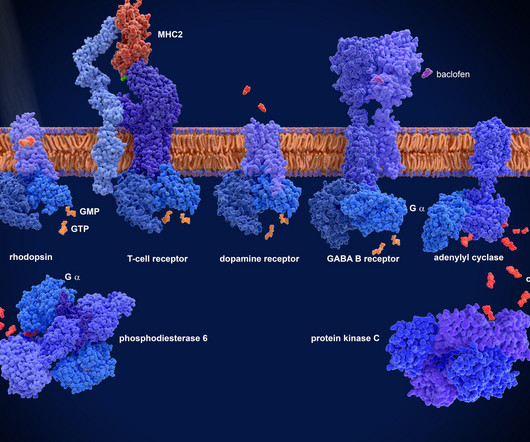
GPCRs are critical targets for drug development due to their involvement in numerous disease pathologies, with many medications working by either activating or inhibiting these receptors. 2022) Why 90% of clinical drug development fails and how to improve it?, References Sun D, et al. doi:10.1016/j.apsb.2022.02.002.










Let's personalize your content