Analysis Life Sciences Thank You Updated guidance on promotional labeling for biosimilars and interchangeables emphasizes a similar approach
Agency IQ
APRIL 26, 2024
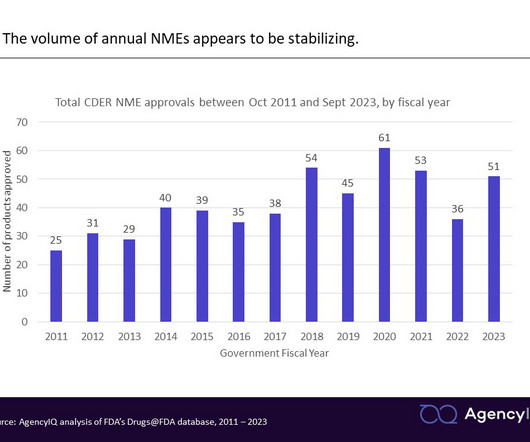
Updated guidance on promotional labeling for biosimilars and interchangeables emphasizes a similar approach Today, the FDA issued a revised draft guidance on the development of promotional labeling for biosimilars, reference products, and—newly—interchangeable products. regarding its administration, preparation, storage, or safety).













Let's personalize your content