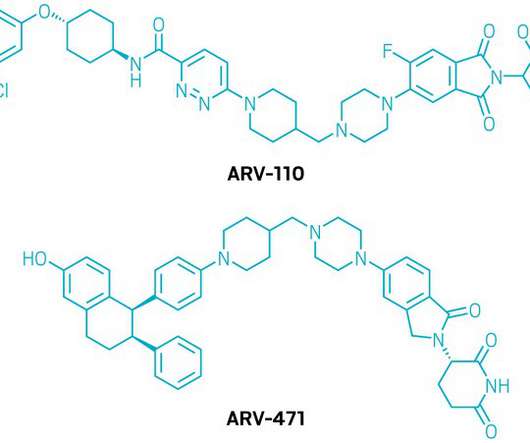
From siloed data to breakthroughs: multimodal AI in drug discovery
Drug Target Review
JUNE 11, 2025
About the authors Remco Jan Geukes Foppen , PhD, is an AI and life sciences expert specialising in the pharmaceutical sector. He holds a master’s degree from University of Salerno in political sciences and marketing. Using clinical genomics and AI in drug development to elevate success.













































Let's personalize your content