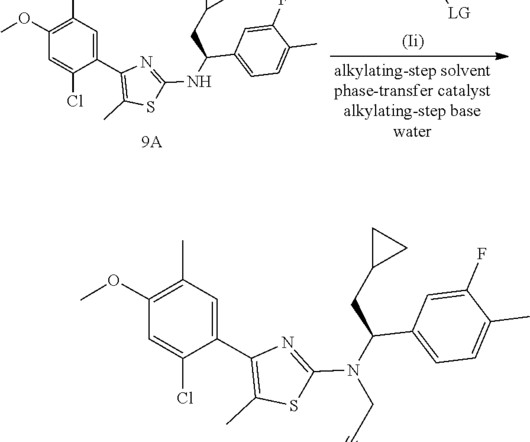
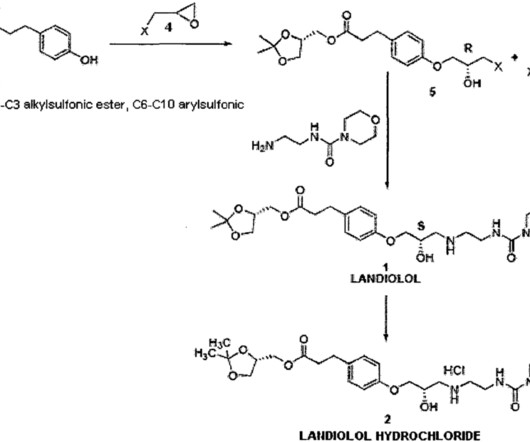
FDA Approves Tablet Form of BeOne’s BRUKINSA® for All Indications
The Pharma Data
JUNE 11, 2025
FDA Approves Tablet Formulation of BeOne’s BRUKINSA® for All Approved Indications, Offering Greater Convenience for Patients with B-cell Cancers BeOne Medicines Ltd. Food and Drug Administration (FDA). a global oncology-focused biopharmaceutical company, has received a significant regulatory milestone from the U.S.





















Let's personalize your content