FDA Approves Merck’s ENFLONSIA™ to Prevent RSV in Infants
The Pharma Data
JUNE 9, 2025
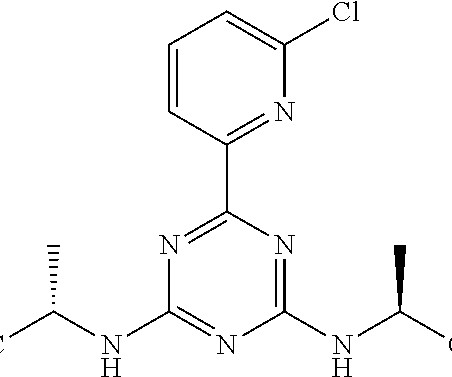
FDA Approves Merck’s ENFLONSIA™ to Protect Infants from Severe RSV Illness Merck operating as MSD outside the United States and Canada, has received a significant regulatory milestone with the U.S. Food and Drug Administration (FDA) granting approval for ENFLONSIA™ (clesrovimab-cfor).








































Let's personalize your content