Elacestrant
New Drug Approvals
MAY 9, 2025
1] [8] Pharmacokinetics Elacestrant has an oral bioavailability of approximately 10%. [1] 2] The FDA granted the application for elacestrant priority review and fast track designations. [2] Food and Drug Administration (FDA). 1] Its plasma protein binding exceeds 99% and remains independent of concentration. [1] 8 February 2023.


















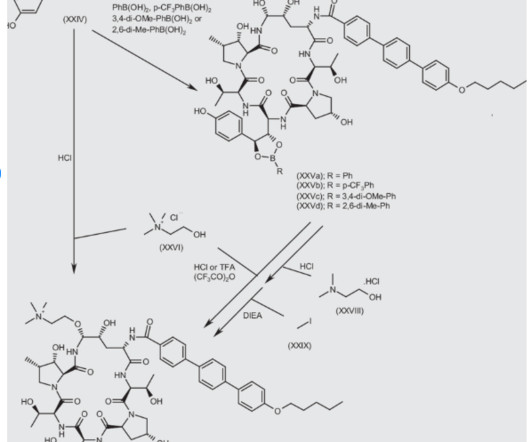
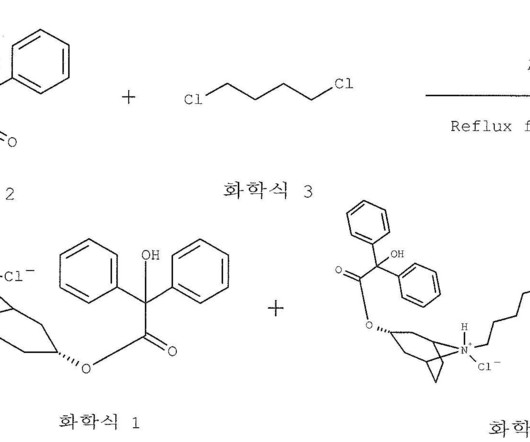

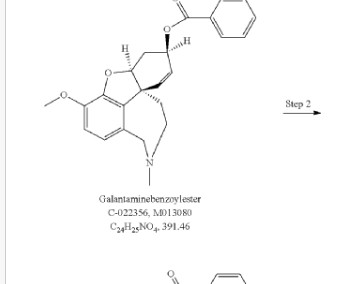
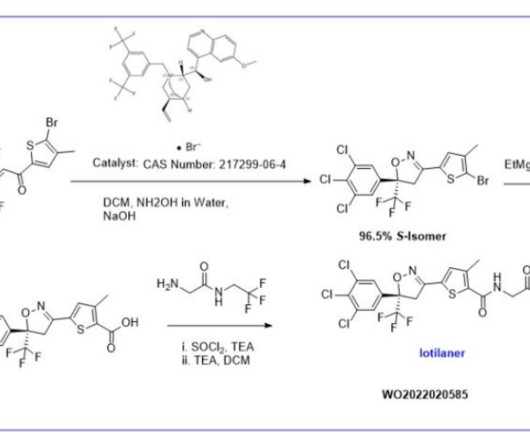







Let's personalize your content