How GPCR agonists, including antibodies, are shaping the future of metabolic care
Drug Target Review
DECEMBER 4, 2024
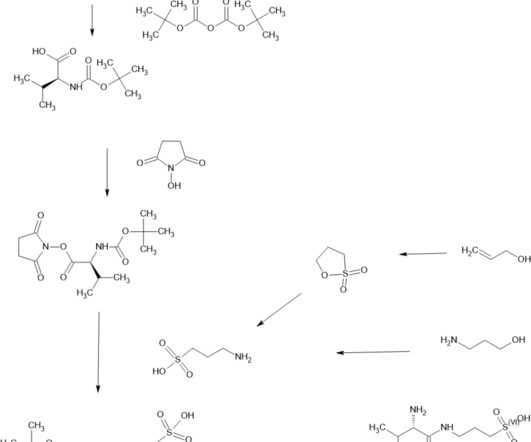
In addition to generating agonistic antibodies, Confo is also successfully applying the ConfoBody/ConfoChimer technology to drugging GPCR with small molecules. A suitable pharmacokinetics profile can be achieved with the addition of half-life extending moieties to the molecule.































Let's personalize your content