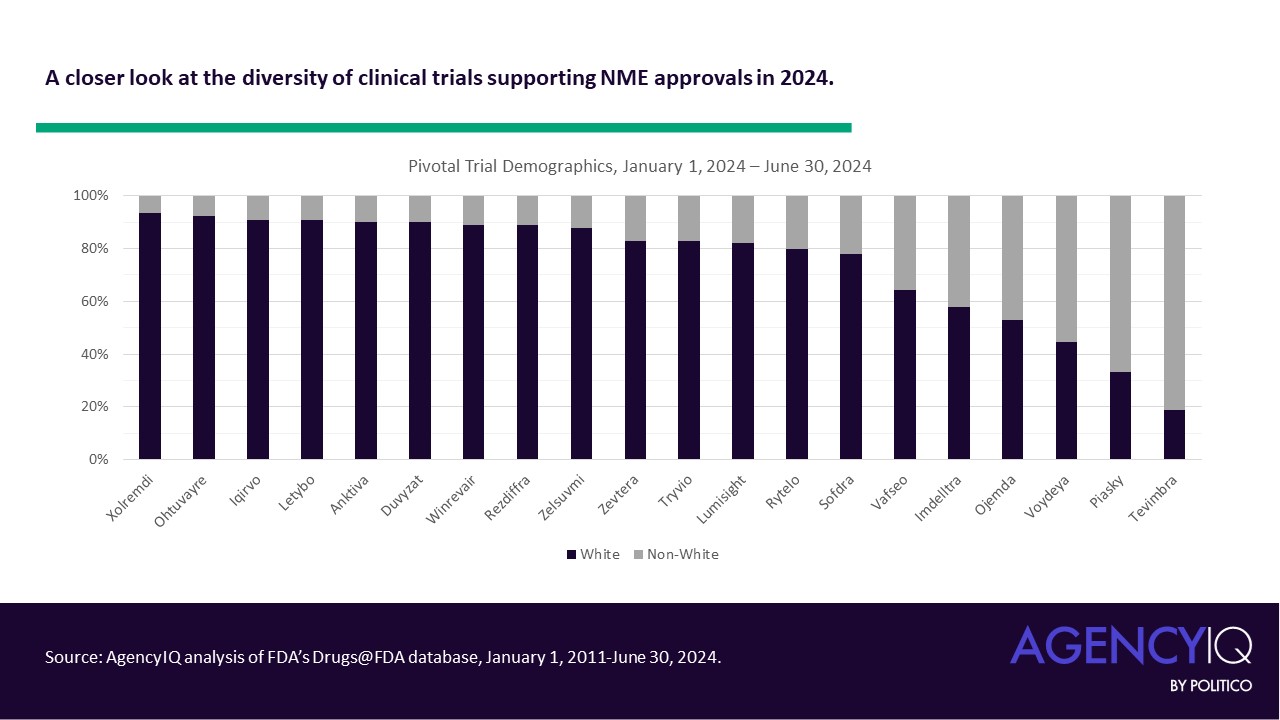
| Drug Name |
Active Ingredient |
Approval Date |
Indication |
| Ohtuvayre |
ensifentrine |
6/26/24 |
To treat chronic obstructive pulmonary disease |
| Piasky |
crovalimab-akkz |
6/20/24 |
To treat paroxysmal nocturnal hemoglobinuria |
| Sofdra |
sofpironium |
6/18/24 |
To treat primary axillary hyperhidrosis |
| Iqirvo |
elafibranor |
6/10/24 |
To treat primary biliary cholangitis in combination with ursodeoxycholic acid |
| Rytelo |
imetelstat |
6/6/24 |
To treat low- to intermediate-1 risk myelodysplastic syndromes |
| Imdelltra |
tarlatamab-dlle |
5/16/24 |
To treat extensive stage small cell lung cancer |
| Xolremdi |
mavorixafor |
4/26/24 |
To treat WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) |
| Ojemda |
tovorafenib |
4/23/24 |
To treat relapsed or refractory pediatric low-grade glioma |
| Anktiva |
nogapendekin alfa inbakicept-pmln |
4/22/24 |
To treat bladder cancer |
| Lumisight |
pegulicianine |
4/17/24 |
To use as an optical imaging agent for the detection of cancerous tissue |
| Zevtera |
ceftobiprole medocaril sodium |
4/3/24 |
To treat certain bloodstream infections, bacterial skin and associated tissue infections, and community-acquired bacterial pneumonia |
| Voydeya |
danicopan |
3/29/24 |
To treat extravascular hemolysis with paroxysmal nocturnal hemoglobinuria |
| Vafseo |
vadadustat |
3/27/24 |
To treat anemia due to chronic kidney disease |
| Winrevair |
sotatercept-csrk |
3/26/24 |
To treat pulmonary arterial hypertension |
| Duvyzat |
givinostat |
3/21/24 |
To treat Duchenne muscular dystrophy in individuals aged 6 years and older |
| Tryvio |
aprocitentan |
3/19/24 |
To treat hypertension |
| Rezdiffra |
resmetirom |
3/14/24 |
To treat noncirrhotic non-alcoholic steatohepatitis with moderate to advanced liver scarring |
| Tevimbra |
tislelizumab-jsgr |
3/13/24 |
To treat unresectable or metastatic esophageal squamous cell carcinoma |
| Letybo |
letibotulinumtoxinA-wlbg |
2/29/24 |
To temporarily improve the appearance of moderate-to-severe glabellar lines |
| Exblifep |
cefepime, enmetazobactam
cefepime, enmetazobactam |
2/22/24 |
To treat complicated urinary tract infections |
| Zelsuvmi |
berdazimer |
1/5/24 |
To treat molluscum contagiosum |