Landiolol
New Drug Approvals
APRIL 19, 2025
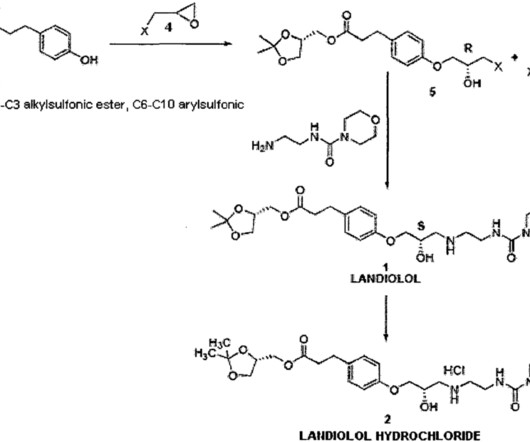
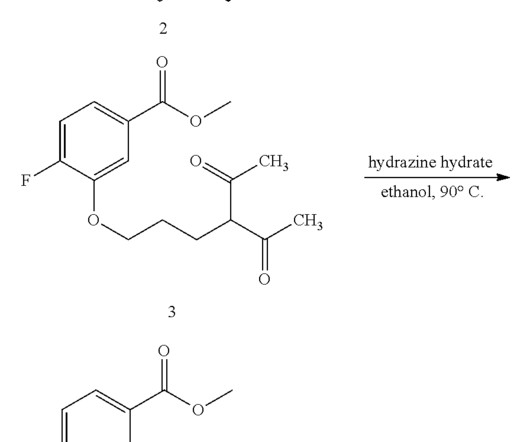
Landiolol 133242-30-5 ONO-1101 Ono 1101 WHO 7516 FDA APPROVED 11/22/2024, Rapiblyk , To treat supraventricular tachycardia C25H39N3O8 509.6 “Pharmacokinetics of landiolol hydrochloride, a new ultra-short-acting beta-blocker, in patients with cardiac arrhythmias” Clinical Pharmacology and Therapeutics.












Let's personalize your content