Cracking the Biosimilar Code: A Deep Dive into Effective IP Strategies
Drug Patent Watch
JULY 22, 2024
In the rapidly evolving landscape of biosimilars, intellectual property (IP) strategy is paramount. Biosimilar manufacturers face unique challenges, including navigating […] Source















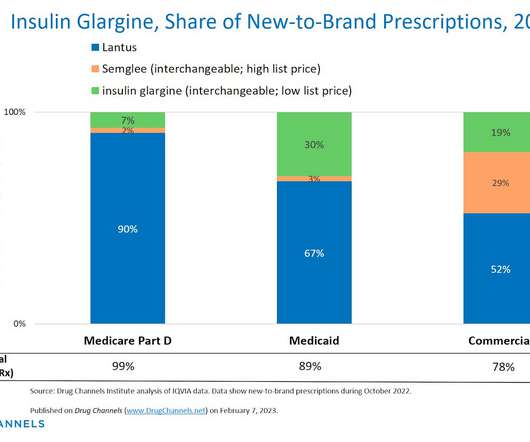
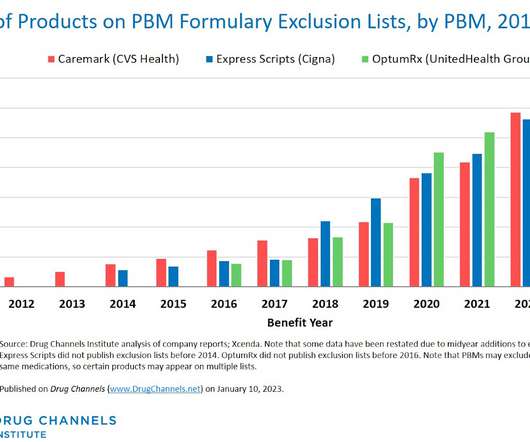
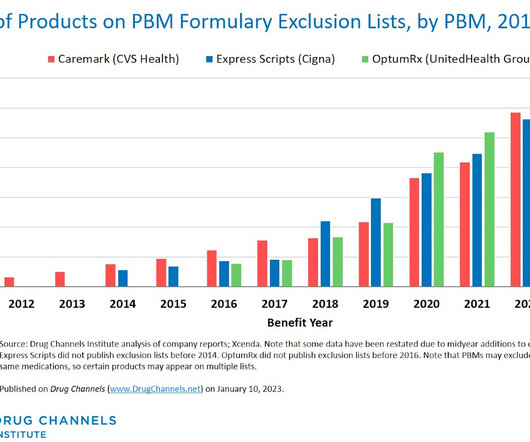
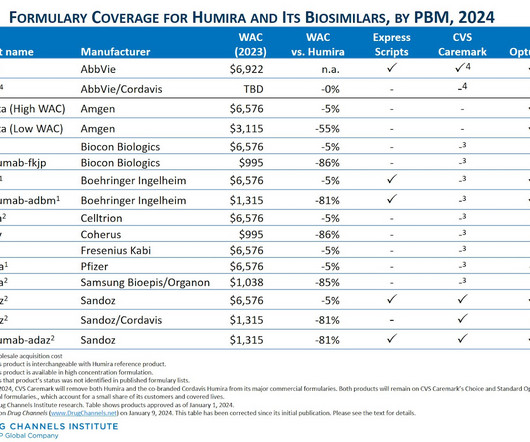




























Let's personalize your content