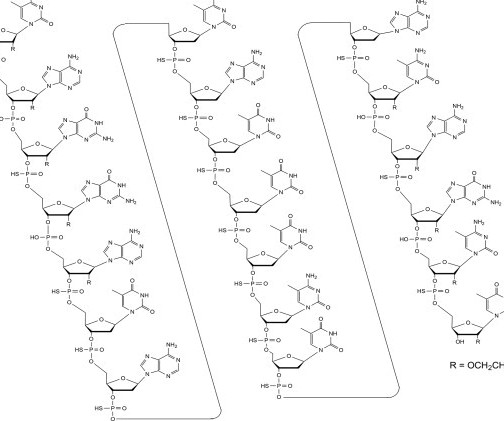
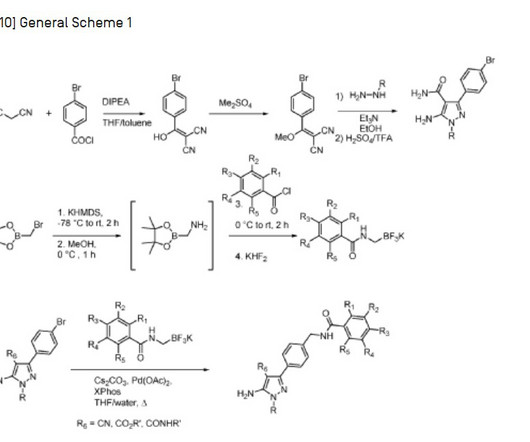
FDA Approves Polypill Widaplik for Hypertension
Drugs.com
JUNE 11, 2025
Food and Drug Administration has approved Widaplik (telmisartan, amlodipine, and indapamide) for the treatment of hypertension in adults.The combination pill is the first and only FDA-approved triple combination.
















































Let's personalize your content