Vamorolone
New Drug Approvals
JULY 25, 2025
7 (2021) 13. [70] 7 (2021) 13 Chemistry Vamorolone is a synthetic corticosteroid and is also known by the chemical name 17α,21-dihydroxy-16α-methylpregna-1,4,9(11)-triene-3,20-dione or as 16α-methyl-9,11-dehydroprednisolone. . Goemans, S. Mercuri, A. Aartsma-Rus, Duchenne muscular dystrophy, Nat. Guglieri, P.R. Clemens, S.J.


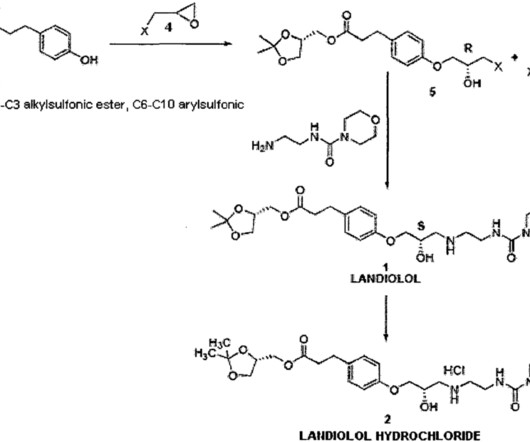


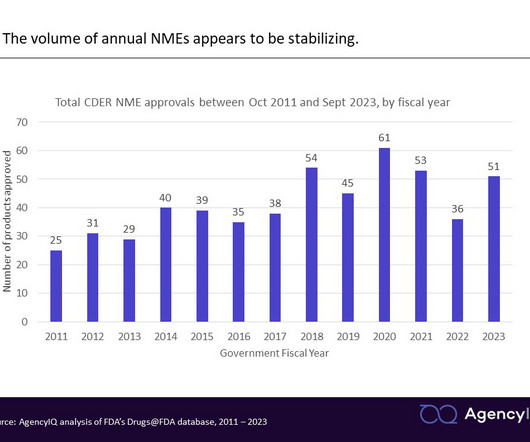








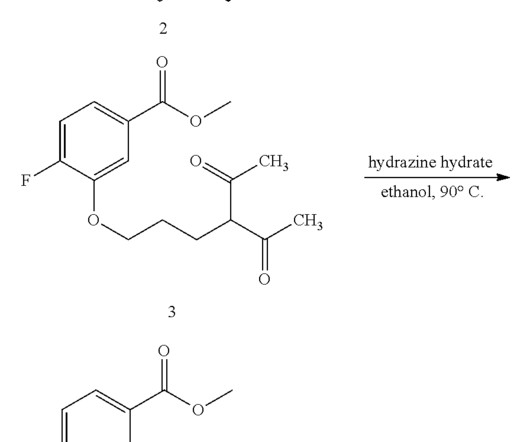






Let's personalize your content