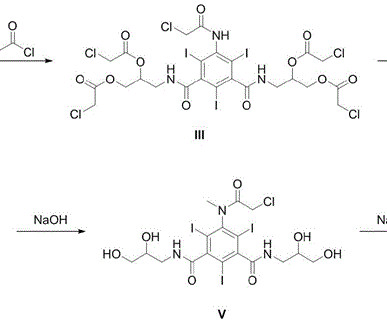
Vamorolone
New Drug Approvals
JULY 25, 2025
2025, 68, 2147−2182 Vamorolone (Agamree). “Population Pharmacokinetics of Vamorolone (VBP15) in Healthy Men and Boys With Duchenne Muscular Dystrophy” Journal of Clinical Pharmacology. Retrieved 11 February 2025. Nagaraju, J.M. Damsker, J.M. 21 (2013) 2241–2249. 77 Readily available steroid 9.1 PMC 6548694.















Let's personalize your content