Targeting HER3 – a little wave of drug development that’s about to get a lot bigger
SugarCone Biotech
JULY 6, 2025
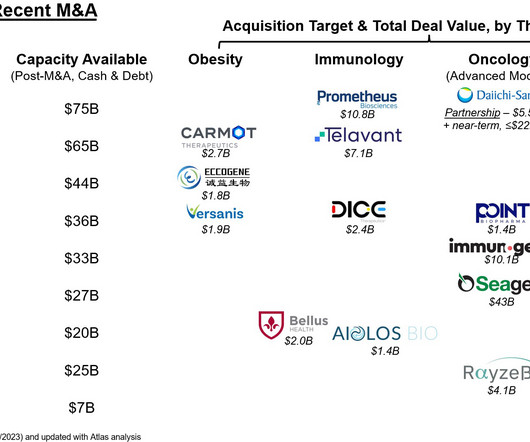
It follows that HER3-targeting therapies have emerged as a class of anti-cancer treatments designed to treat various tumors that have developed resistance to EGFR or HER2-directed therapies or have emerged due to a NRG1 gene fusion. This is an active drug development landscape with a lot of recent news.












Let's personalize your content