Analysis Life Sciences Thank You What We Expect the FDA to do in July and August 2024
Agency IQ
JULY 8, 2024
and the E.C.

Agency IQ
JULY 8, 2024
and the E.C.
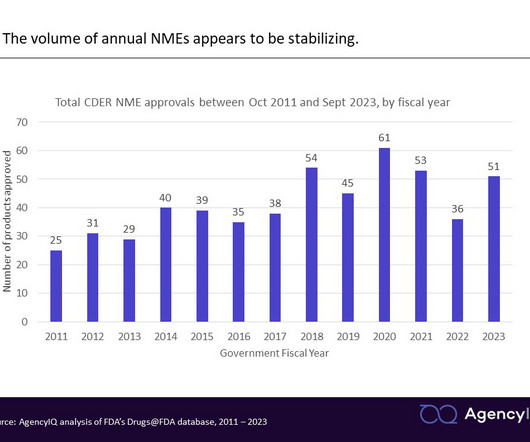
Agency IQ
OCTOBER 6, 2023
CDER is the FDA office in charge of reviewing pharmaceuticals and therapeutic biologics. All products are received in one of two forms: A New Drug Application (or NDA, for pharmaceuticals) or a Biologics License Application (BLA, for biologics). As AgencyIQ has previously discussed , developing biosimilars is an expensive process.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Agency IQ
FEBRUARY 12, 2024
Accelerated Approval of Drugs and Biologics Administrative/ Procedural New Civil Monetary Penalties for Failure to Meet Accelerated Post Marketing Requirements Administrative/ Procedural Carried over from previous guidance agenda Exclusivity for First Interchangeable Biosimilar Biological Products Administrative/ Procedural Carried over from previous (..)

Agency IQ
JUNE 2, 2023
6/27/2023 Notification FDORA, Section 3201 Within 180 days of the passage of FDORA, all biologics and biosimilars sponsors must submit a written notice to the FDA of all actively marketed products (i.e., These inspections will be routine surveillance rather than inspections as part of an approval application.

Agency IQ
FEBRUARY 15, 2024
We have tried to sort guidance documents by topic area.

Agency IQ
OCTOBER 27, 2023
WUSF / AgencyIQ November 1 Initial deadline for NDSRIs Under a 2023 guidance document, the FDA has recommended that pharmaceutical companies assess Nitrosamine Drug-Related Substance Impurities for their products by November 1, 2023, with confirmatory testing due by August 1, 2025.

Agency IQ
MAY 3, 2024
Title Type Comments Close Key Information and Facilitating Understanding in Informed Consent Guidance for Sponsors, Investigators, and Institutional Review Boards Draft Guidance April 30 Early Alzheimer’s Disease: Developing Drugs for Treatment Draft Guidance May 13 Select Updates for the Premarket Cybersecurity Guidance: Section 524B of the (..)
Let's personalize your content