Time for change: non-human primates in drug research
Drug Target Review
JULY 7, 2025
Their unique suitability has made them valuable for evaluating pharmacokinetics, toxicology and safety in drug candidates before human clinical trials.

Drug Target Review
JULY 7, 2025
Their unique suitability has made them valuable for evaluating pharmacokinetics, toxicology and safety in drug candidates before human clinical trials.

FDA Law Blog: Drug Discovery
FEBRUARY 2, 2023
Since 1962, the FD&C Act has authorized FDA to require that sponsors of clinical trials submit data from “preclinical tests (including tests on animals)” in order to demonstrate that their drug is safe enough to advance to testing in humans. For more on FDORA’s other provisions, see HPM’s complete summary here ). 42 U.S.C. §
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

DrugBank
FEBRUARY 12, 2025
This approach capitalizes on prior investments in R&D, mitigates risk by leveraging established safety and pharmacokinetic profiles, and accelerates the delivery of treatments to patients. Key techniques include high-throughput screening (HTS), cell-based assays, and animal models.
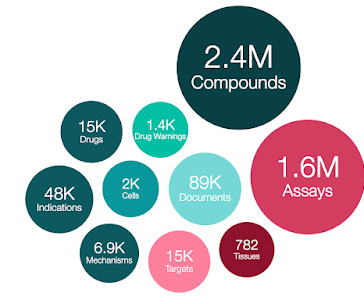
The ChEMBL-og
APRIL 16, 2024
See [link] and INN clinical candidate drugs (assigned as max_phase = 2 based on INN guidance that states “As a general guide, the development of a drug should progress up to the point of clinical trials (phase II) before an application is submitted to the INN Secretariat for name selection.” University of Dundee: T.

Alta Sciences
SEPTEMBER 27, 2024
Featuring two scenarios that explore the complexities of bioanalysis for immunomodulators, The Altascientist offers practical considerations for ensuring accurate bioanalysis, as well as pharmacokinetic, pharmacodynamic , and safety data in clinical trials.
Let's personalize your content