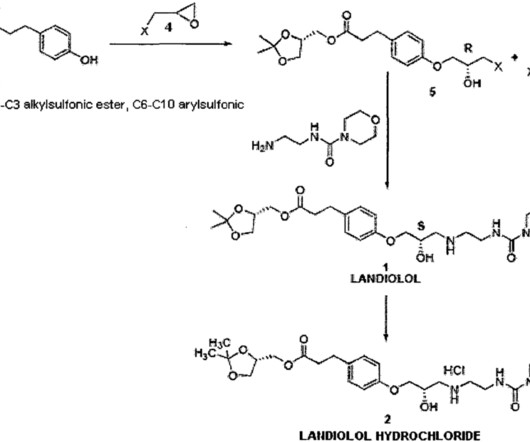
Landiolol
New Drug Approvals
APRIL 19, 2025
The fast turnover of landiolol will diminish most adverse events due to self-limiting administration. Landiolol prophylaxis is associated with reduced incidence of postoperative atrial fibrillation without triggering adverse events related to a beta-blockade. Atarashi H, Kuruma A, Yashima M, Saitoh H, Ino T, Endoh Y, et al.

















Let's personalize your content