Vamorolone
New Drug Approvals
JULY 25, 2025
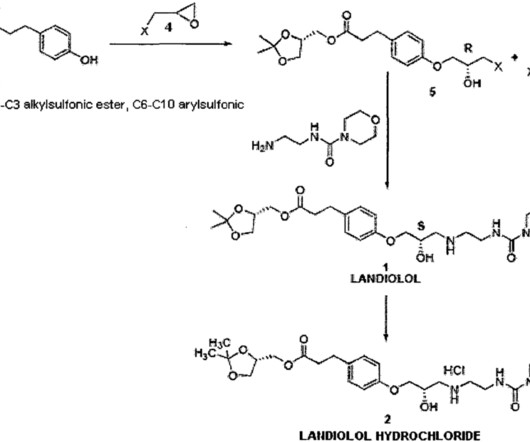
Corticosteroid therapy is the current standard of care for DMD despite relatively high rates of adverse effects. 75,76 This also results in decreased glucocorticoid receptor-drive transactivation, ultimately improving the safety profile of vamorolone as compared to other corticosteroid therapies. 77 Readily available steroid 9.1




















Let's personalize your content