Tofersen
New Drug Approvals
MAY 17, 2025
3] Tofersen was approved for medical use in the United States in April 2023, [3] [6] and in the European Union in May 2024. [4] 4] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [7] gmol 1 References ^ “Register of Innovative Drugs” Health Canada. 3 November 2006.

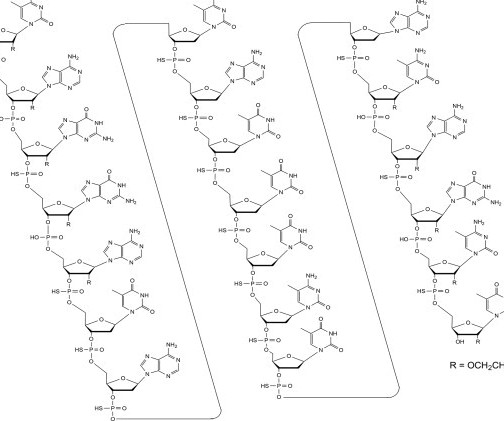


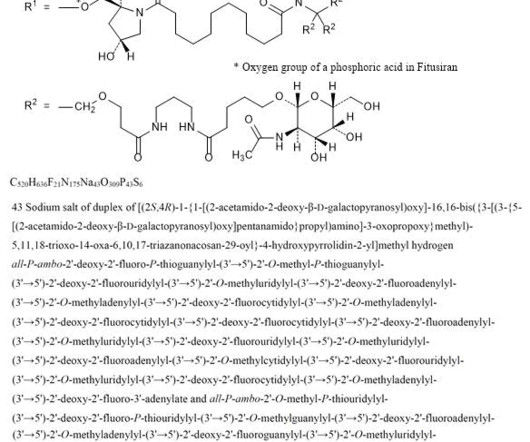

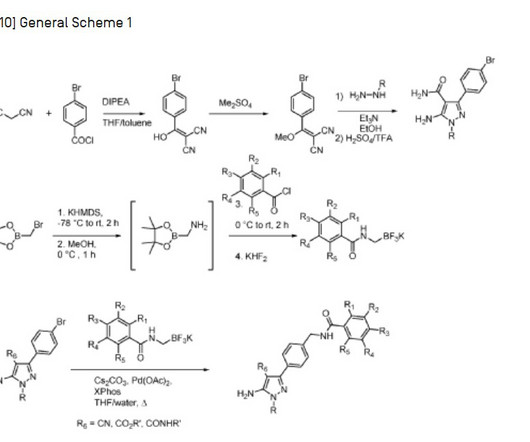
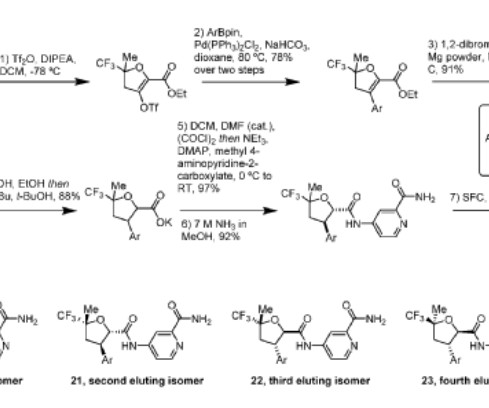




































Let's personalize your content