Identification of a phenyl ester covalent inhibitor of caseinolytic protease and analysis of the ClpP1P2 inhibition in mycobacteria
Covalent Modifiers
APRIL 20, 2025
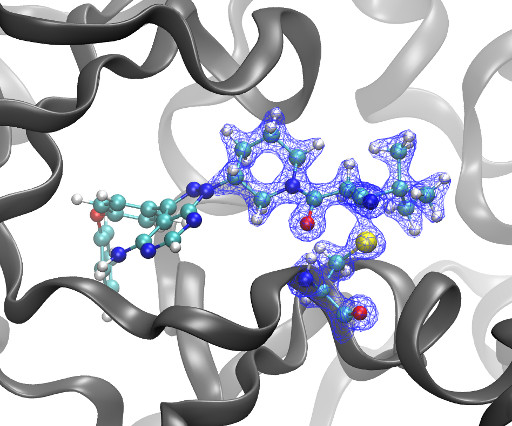
Genhui Xiao, Yumeng Cui, Liangliang Zhou, Chuya Niu, Bing Wang, Jinglan Wang, Shaoyang Zhou, Miaomiao Pan, Chi Kin Chan, Yan Xia, Lan Xu, Yu Lu, Shawn Chen mLife , 2025 DOI: [link] The caseinolytic protease complex ClpP1P2 is crucial for protein homeostasis in mycobacteria and stress response and virulence of the pathogens. Its role as a potential drug target for combating tuberculosis (TB) has just begun to be substantiated in drug discovery research.










Let's personalize your content