Chimeric deubiquitinase engineering reveals structural basis for specific inhibition of the mitophagy regulator USP30
Covalent Modifiers
MAY 18, 2025
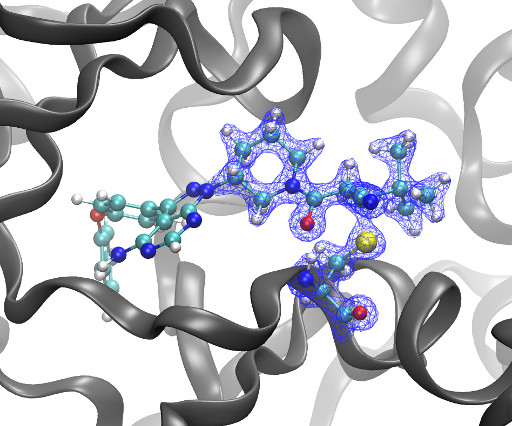
Nafizul Haque Kazi, Nikolas Klink, Kai Gallant, Gian-Marvin Kipka & Malte Gersch Nat Struct Mol Biol , 2025 [link] The mitochondrial deubiquitinase ubiquitin-specific protease (USP) 30 negatively regulates PINK1parkin-driven mitophagy. Whether enhanced mitochondrial quality control through inhibition of USP30 can protect dopaminergic neurons is currently being explored in a clinical trial for Parkinsons disease.











Let's personalize your content