U.S. CDC Advisory Committee on Immunization Practices Recommends Vaccination with Moderna’s COVID-19 Vaccine for Persons 18 Years and Older
The Pharma Data
DECEMBER 19, 2020
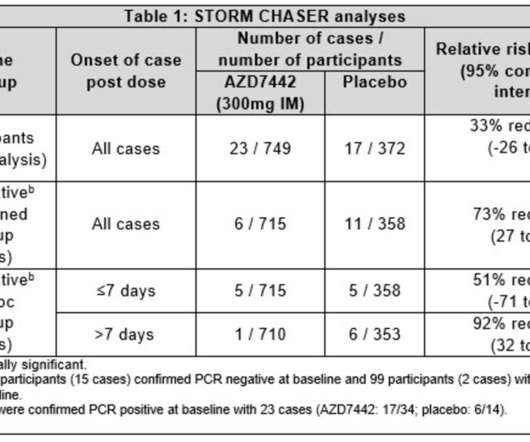
Today’s ACIP recommendation follows the December 1, 2020 ACIP recommendation for a Phase 1a rollout in which the first priority for COVID-19 vaccines is given to healthcare personnel treating patients and residents in long-term care facilities. Department of Health and Human Services (HHS) for review and adoption.














Let's personalize your content