Regulatory Guidance for Oligonucleotide Bioanalysis in Drug Development
Alta Sciences
FEBRUARY 19, 2025
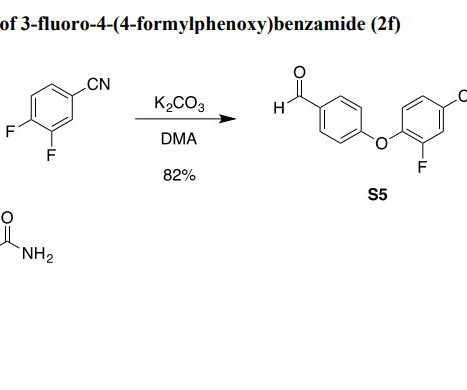
Regulatory Guidance for Oligonucleotide Bioanalysis in Drug Development pmjackson Wed, 02/19/2025 - 21:30 The unique physicochemical properties of oligonucleotides require the use of specialized bioanalytical approaches, with key considerations including selectivity and specificity, sensitivity, stability, and matrix effects.



















Let's personalize your content