N-glucuronidation: the human element
Metabolite Tales Blog
DECEMBER 13, 2023
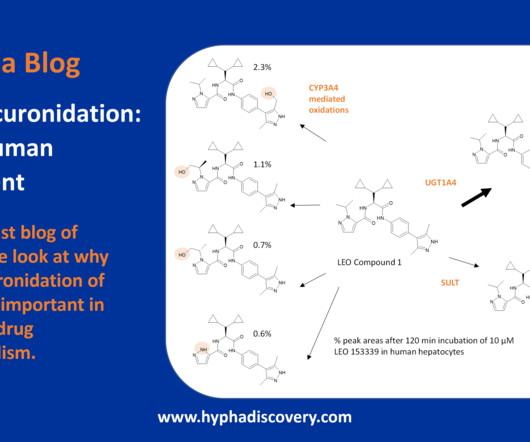
N -glucuronidation: the human element By Julia Shanu-Wilson In our last blog of the year, we look at why N -glucuronidation of drugs is important in human drug metabolism. Glucuronidation is the most common phase II reaction observed in the metabolism of drugs in humans. Xuan Qin, Yong Wang, Kevin R. MacKenzie, John M.







Let's personalize your content