Gepirone
New Drug Approvals
JUNE 11, 2025
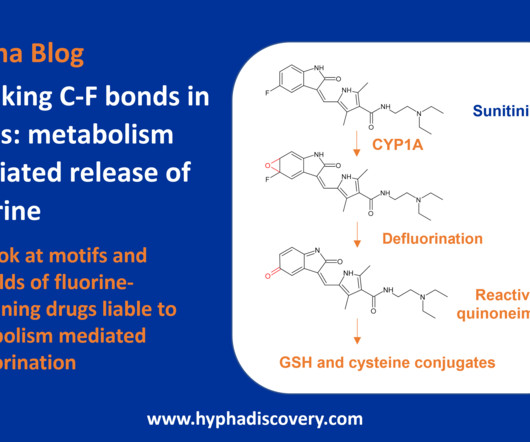
2] Gepirone was synthesized by Bristol-Myers Squibb in 1986 and was developed and marketed by Fabre-Kramer Pharmaceuticals. [4] 4] It was approved for the treatment of major depressive disorder in the United States in September 2023. [4] 12] However, in March 2016, the FDA reversed its decision and gave gepirone ER a positive review.























Let's personalize your content