How to Identify Branded Drugs with a Low Likelihood of Generic Entry as Targets for In-Licensing
Drug Patent Watch
DECEMBER 12, 2024
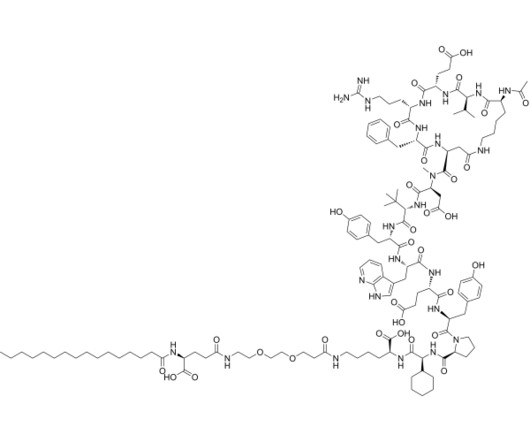
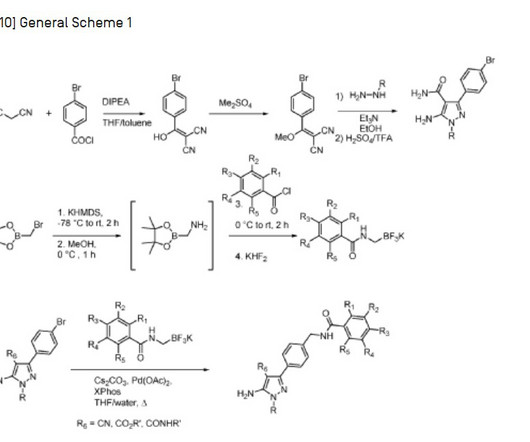
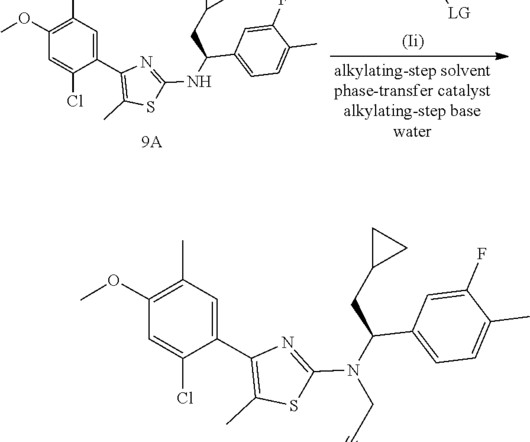
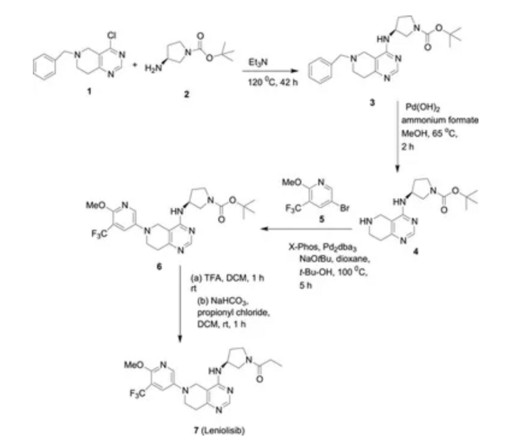
Identifying branded drugs with a low likelihood of generic entry has become a crucial strategy for companies looking to expand their product portfolio through in-licensing. In this comprehensive guide, we’ll explore the intricacies of identifying such drugs and leveraging them for successful in-licensing opportunities.















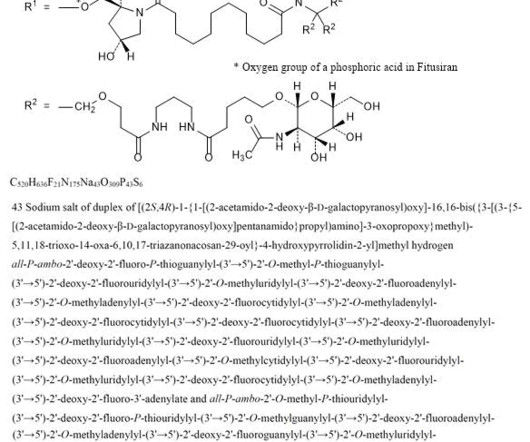
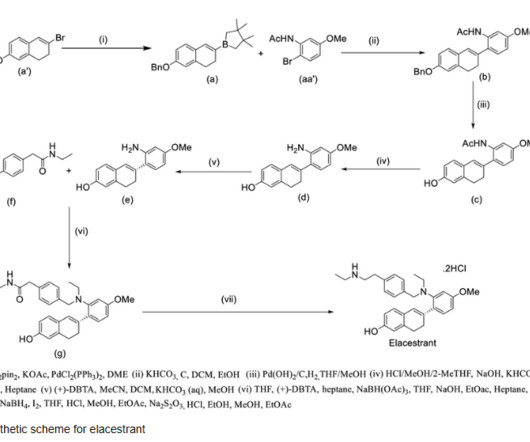



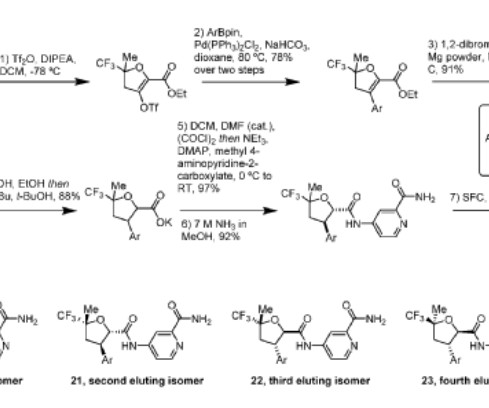
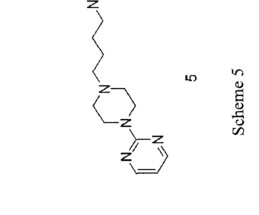


















Let's personalize your content