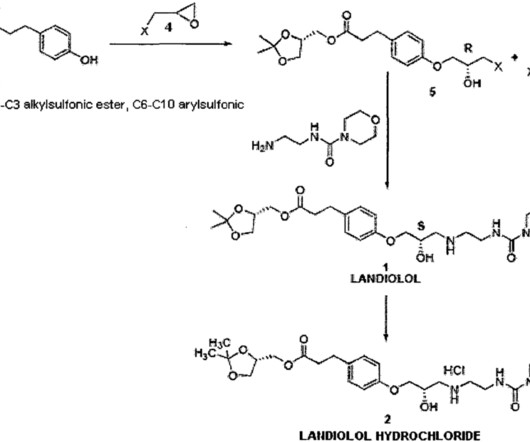
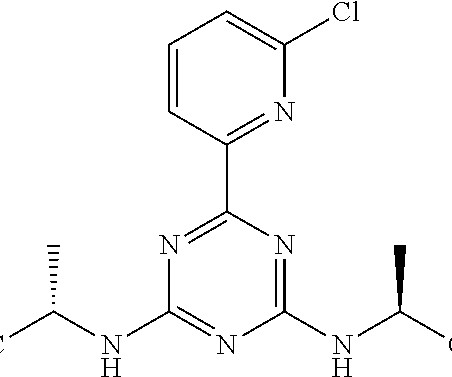
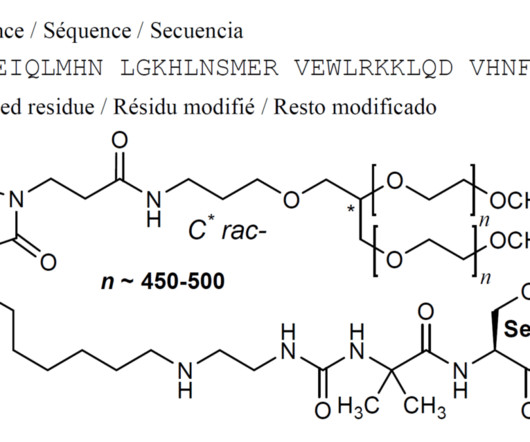
Tofersen
New Drug Approvals
MAY 17, 2025
3] Tofersen was approved for medical use in the United States in April 2023, [3] [6] and in the European Union in May 2024. [4] 4] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [7] Food and Drug Administration (FDA). Jump up to: a b New Drug Therapy Approvals 2023 (PDF).




































Let's personalize your content