Targeting HER3 – a little wave of drug development that’s about to get a lot bigger
SugarCone Biotech
JULY 6, 2025
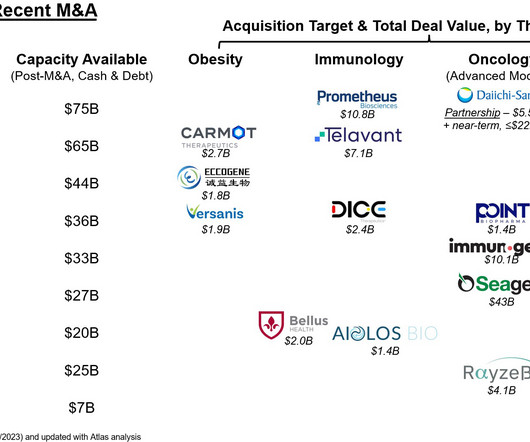
They have interesting patterns of expression in different cancer indications; thus, diverse therapies for attacking these targets have been developed. These results are notable given the poor response of NRG1 fusion driven cancers to chemo- and immuno-therapies. These activities are pro-oncogenic (reviewed here: [link] ).















Let's personalize your content