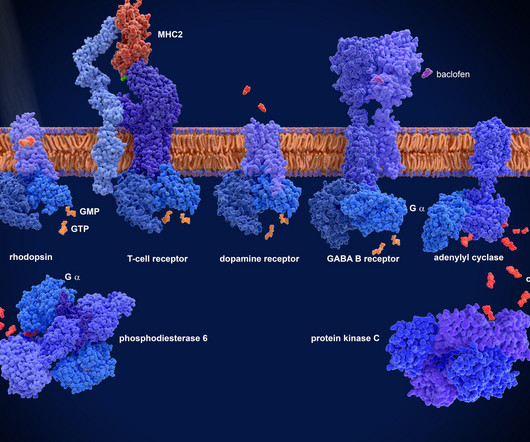
Advancements in hit identification for membrane protein drug discovery
Drug Target Review
APRIL 7, 2025
Advancements in screening technologies for small-molecule drug discovery including cellular assays, computational screening, and biophysics-based methods enhanced by structural biology breakthroughs have improved screening hit rates and facilitated the identification of drug candidates for previously undruggable targets.














Let's personalize your content