How GPCR-targeting therapies are advancing the fight against inflammatory disease
Drug Target Review
JUNE 16, 2025
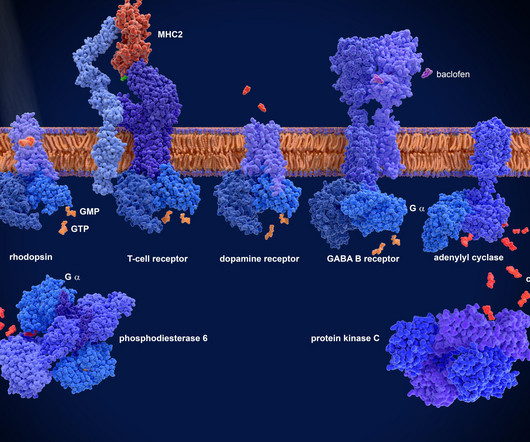
However, due to its unique biology and structural complexity, PAR2 has proven notoriously difficult for the pharmaceutical industry to effectively target. DT-9046 is currently progressing through pre-IND studies, supported by a robust data package, including head-to-head benchmarks and strong patent protection.



























Let's personalize your content