Analyzing biosimilar market dynamics in different patient populations
Drug Patent Watch
JANUARY 13, 2025
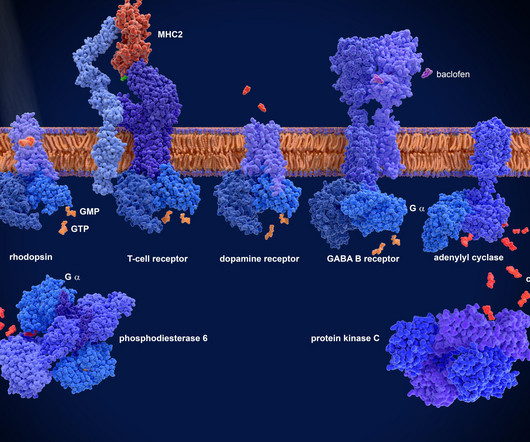
Biosimilars have emerged as a game-changing force, promising to revolutionize patient access to life-saving biologics while simultaneously reducing healthcare costs. ”[1] The global biosimilars market is experiencing exponential growth, with projections indicating it will reach $69.4 billion by 2025, growing at a CAGR of 34.2%














































Let's personalize your content