Bio-Techne Joins Forces with USP to Speed Up Development of mAbs and Gene Therapies
The Pharma Data
JUNE 24, 2025
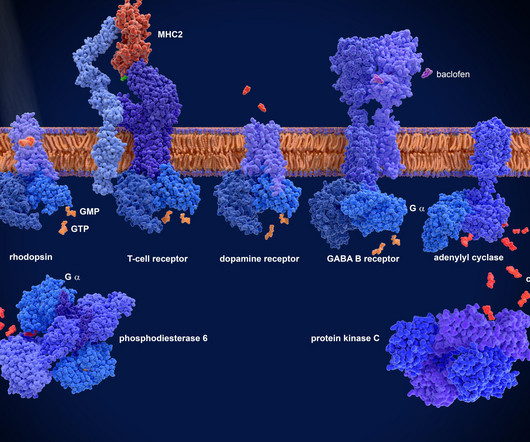
This alliance aims to address mounting challenges in the development and commercialization of mAb therapies and gene therapies, particularly those involving AAV vectors. To date, more than 160 monoclonal antibody therapies targeting nearly 100 disease-related proteins have received regulatory approval globally.













































Let's personalize your content