The rising impact of biomarkers in early clinical development
Drug Target Review
APRIL 7, 2025
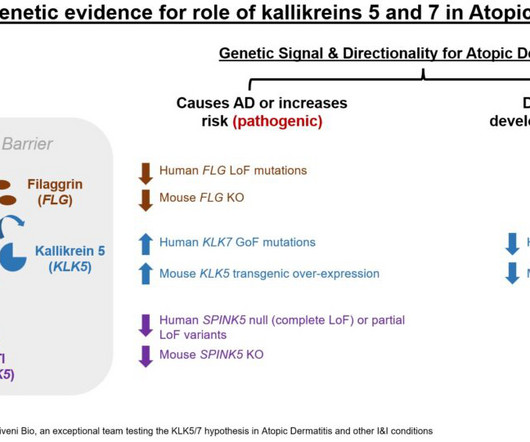
As pharmacological indicators, biomarkers overcome the static nature of traditional in vitro cellular studies by providing more dynamic models of pharmacokinetic processes that reflect active biological mechanisms. Biomarkers can play a crucial role throughout clinical development, especially in early phases.









































Let's personalize your content