The rising impact of biomarkers in early clinical development
Drug Target Review
APRIL 7, 2025
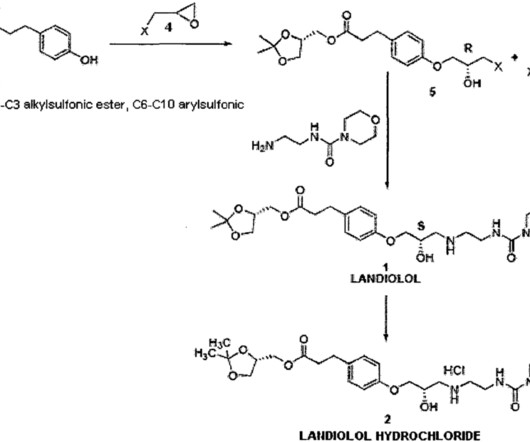
While biomarkers are increasingly being adopted as surrogate endpoints by the US Food and Drug Administration (FDA), advances in biomarker detection also present a variety of regulatory challenges related to the complexities in their selection, implementation and clinical interpretation. References FDA-NIH Biomarker Working Group.









































Let's personalize your content