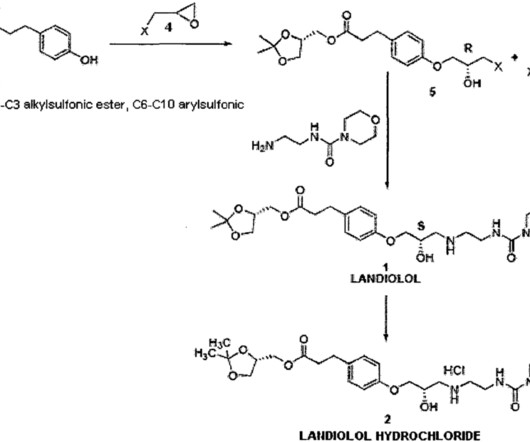
Vamorolone
New Drug Approvals
JULY 25, 2025
“Agamree Product information” Union Register of medicinal products. “Population Pharmacokinetics of Vamorolone (VBP15) in Healthy Men and Boys With Duchenne Muscular Dystrophy” Journal of Clinical Pharmacology. Retrieved 27 December 2023. Reproduction is authorized provided the source is acknowledged.

























Let's personalize your content