Vamorolone
New Drug Approvals
JULY 25, 2025
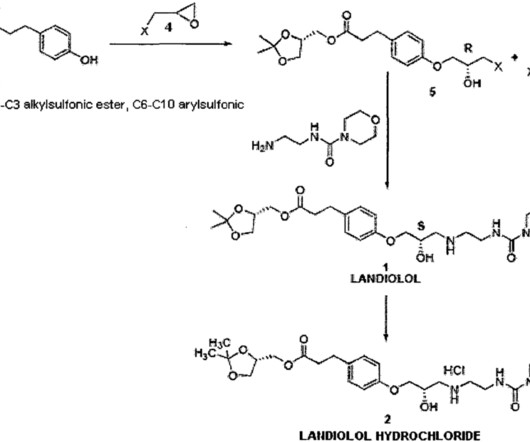
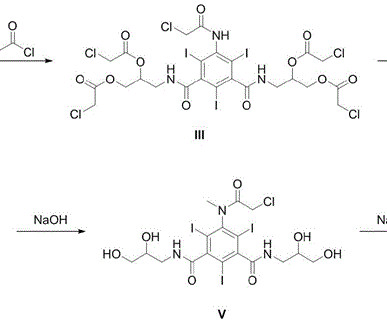
Example 2: Synthesis of the present invention Scheme C: Route of Synthesis of Vamorolone from 8-DM Vamorolone was synthesized in three synthetic steps from commercially available 8-DM. “Population Pharmacokinetics of Vamorolone (VBP15) in Healthy Men and Boys With Duchenne Muscular Dystrophy” Journal of Clinical Pharmacology.





















Let's personalize your content