Elacestrant
New Drug Approvals
MAY 9, 2025
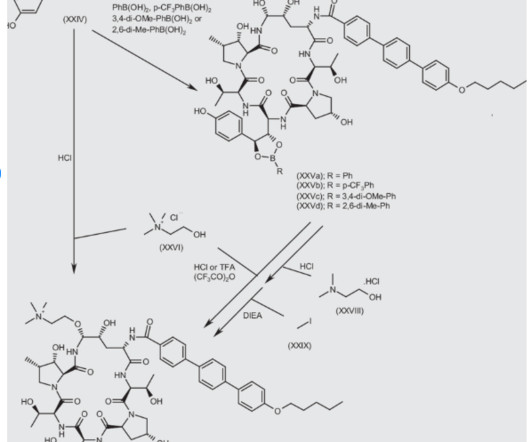
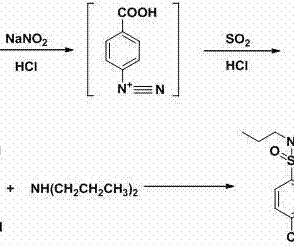
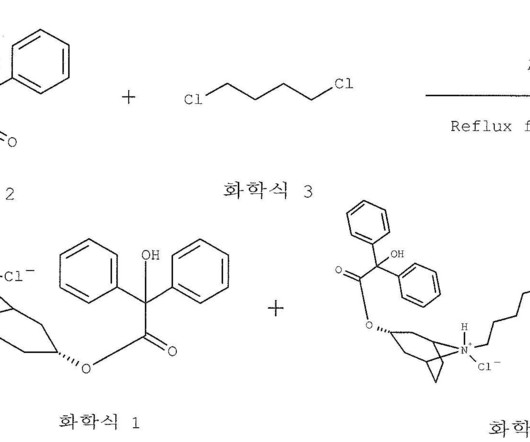
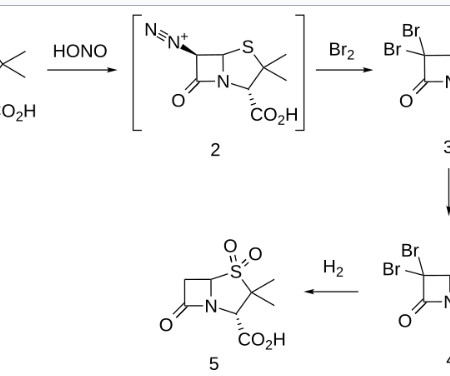
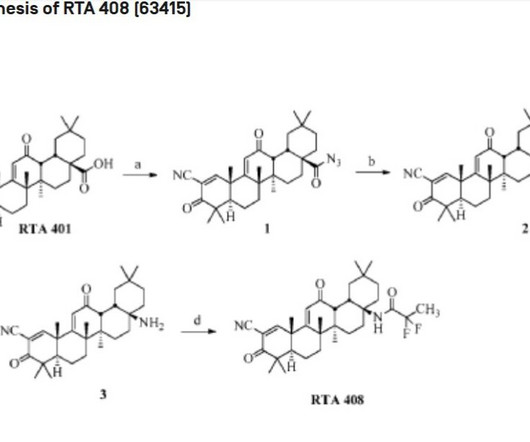
1] [8] Pharmacokinetics Elacestrant has an oral bioavailability of approximately 10%. [1] 1] [4] It is taken by mouth. [1] 1] [4] Elacestrant is an antiestrogen that acts as an antagonist of estrogen receptors , which are the biological targets of endogenous estrogens like estradiol. [1] 3] [7] PATENTS Cruskie MP, et al.











Let's personalize your content