Medicines Manufacturing Innovation Centre set to Transform UK Pharma
Drug Discovery Today
AUGUST 23, 2021
Medicines Manufacturing Innovation Centre in Renfrewshire, Scotland, has topped out.

Drug Discovery Today
AUGUST 23, 2021
Medicines Manufacturing Innovation Centre in Renfrewshire, Scotland, has topped out.

Drug Patent Watch
AUGUST 16, 2021
I’ll be leading a workshop on generic portfolio management, and also giving a talk, at the 14th annual Marcus Evans event on Portfolio Planning and Partnerships for Generics. The event…. The post Upcoming workshop on generic portfolio management appeared first on DrugPatentWatch - Make Better Decisions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Channels
AUGUST 17, 2021
The generic prescription market is being disrupted—but not by the big, bad spaceman from Seattle. Instead, consider how GoodRx is affecting patients, payers, and PBMs. Below I summarize the latest financial results for GoodRx’s discount card business. We estimate that the company accounted for $4.1 billion in U.S. prescription revenues for 2021. That’s about six times its 2016 figure.
The Pharma Data
AUGUST 31, 2021
Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. (NYSE and TASE: TEVA), and MedinCell (Euronext: MEDCL) announced today that the New Drug Application (NDA) for TV-46000/mdc-IRM (risperidone extended-release injectable suspension for subcutaneous use) for the treatment of schizophrenia has been accepted by the U.S. Food and Drug Administration (FDA).

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Quanticate
AUGUST 27, 2021
R is the open source software environment and language used for data analysis and statistical computing. A great deal has already been said and written about R’s wide variety of graphical and statistical techniques. Even though R as a programming language is constantly growing in popularity in the pharmaceutical industry, it is still quite unpopular to use R in the preliminary stages of research like importing data from different sources, tidying it, calculating new variables in datasets and mak
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Drug Discovery Today
AUGUST 20, 2021
• ELRIG Drug Discovery 2021, ACC Liverpool, UK, 19–20 October• Face-to-face event aims to re-connect, re-invent, and re-imagine drug discovery• SLAS’s Innovation AveNEW promotes start-ups by awarding free-of-charge exhibition space and access to a network of potential business partners
Drug Patent Watch
AUGUST 24, 2021
Annual Drug Patent Expirations for SYNJARDY Synjardy is a drug marketed by Boehringer Ingelheim and is included in two NDAs. It is available from one supplier. There are six patents…. The post New patent for Boehringer Ingelheim drug SYNJARDY appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
AUGUST 31, 2021
Charlotte Morabito at CNBC has put together “Why Pharmacies Overcharge,” an entertaining and provocative video on the pharmacy industry and its generic prescription pricing. It’s definitely worth your time. I especially enjoyed the cool visualizations of Drug Channels Institute’s industry data. Links below. The video covers the history of pharmacy, from the “Soda Fountain Era” to “Lick, Stick, and Pour” to the rise of PBMs and GoodRx. .

The Pharma Data
AUGUST 31, 2021
Eli Lilly and Company (NYSE: LLY) will participate in Citi’s 16th Annual BioPharma Virtual Conference on September 8 and 9, 2021. Anat Ashkenazi, senior vice president and chief financial officer, will participate in a virtual fireside chat on Thursday, September 9 at 9:45 a.m., Eastern Time. A live audio webcast will be available on the “Webcasts & Presentations” section of Lilly’s Investor website at [link].

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Crown Bioscience
AUGUST 16, 2021
While the authentication of biosamples (i.e., cell lines, organoids, xenograft and homograft models) has long been recommended, misidentification and contamination remains a problem. In this post, we explore two of the traditional (low throughput) genomic-based assays used for assessing biosample authentication and contamination: short tandem repeat (STR) and single nucleotide polymorphism (SNP) analysis.

The Strateos Blog: Drug Discovery
AUGUST 16, 2021
On July 20th, Strateos hosted a webinar entitled Drug Discovery and Medicinal Chemistry in the Era of Automation. Daniel Rines, Ph.D., Sr. Director of R&D Strategy and Vince Yeh, Ph.D., Director of Chemistry of Strateos gave an interactive talk focused on how automated robotics and integrated software are powering self-driving labs that are revolutionizing synthetic chemistry to support medicinal chemistry workflows.

Drug Discovery Today
AUGUST 18, 2021
A new study, produced in partnership between the Royal Veterinary College (RVC), the Wellcome Sanger Institute, Imperial College and the Vector Borne and Neglected Tropical Disease Control Division of the Ministry of Health, Uganda, has been published in Nature Communications. This research paves the way for a more effective genomic surveillance of schistosomiasis, a major human parasite.
Drug Patent Watch
AUGUST 23, 2021
Annual Drug Patent Expirations for JARDIANCE Jardiance is a drug marketed by Boehringer Ingelheim and is included in one NDA. It is available from four suppliers. There are six patents…. The post New patent for Boehringer Ingelheim drug JARDIANCE appeared first on DrugPatentWatch - Make Better Decisions.
Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Drug Channels
AUGUST 11, 2021
The Medicare Payment Advisory Commission (MedPAC), the independent agency that advises Congress on the Medicare program, recently released its latest Health Care Spending and the Medicare Program databook. This annual report is a treasure trove of useful and fascinating data. The July 2021 edition provides us with the latest pre-pandemic details on the buy-and-bill market in Medicare Part B.
The Pharma Data
AUGUST 31, 2021
today announced a label update for KEYTRUDA, Merck’s anti-PD-1 therapy, for its indication in first-line advanced urothelial carcinoma (bladder cancer) in the U.S. The U.S. Food and Drug Administration (FDA) has converted this indication from an accelerated to a full (regular) approval. In addition, as part of the label update, this indication has been revised to be for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) who are not eligible for any platinum-

Molecule Blog
AUGUST 13, 2021
Announcing the first biopharma IP-NFT Transaction Funding the first longevity research project by utilizing intellectual property NFTs in the pharmaceutical space Summary The vote has passed, the decision is made: The Scheibye-Knudsen Lab will be the first research organisation to fund their longevity research via an IPNFT. This historic moment needs to be celebrated properly!
ProRelix Research
AUGUST 12, 2021
Acceptance of foreign clinical trials data by US FDA Foreign Clinical Trials (FCTs) Prior to the 1962 Kefauver-Harrison Amendments, it was uncommon for a Sponsor to submit FCTs data. In […]. The post Acceptance of foreign clinical trials data by US FDA appeared first on ProRelix Research.
Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Drug Discovery Today
AUGUST 18, 2021
Wallops Flight Facility, VA. August 10, 2021 - An 18-year-old high school graduate has developed an elegant new way to gauge the liver health of astronauts—and it could someday help solve an enduring medical mystery in space.
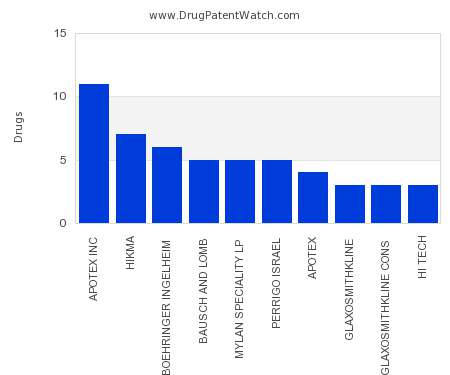
Drug Patent Watch
AUGUST 27, 2021
This chart shows the pharmaceutical companies with the most spray dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most spray dosed drugs…. The post Which pharmaceutical companies have the most spray dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
AUGUST 13, 2021
Today’s guest post comes from Stacey Little, Senior Vice President of Business Development and Marketing at AssistRx. Stacey discusses how a patient support vendor can help specialty pharmacy patients attain prior authorizations and access critical therapies. She offers three criteria that manufacturers can use to evaluate e-support services providers.

The Pharma Data
AUGUST 30, 2021
Metoprolol, a drug widely used to treat cardiovascular disease, is beneficial when administered to COVID-19patients. This is the finding of a study by investigators at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), published today in the Journal of the American College of Cardiology (JACC). The most severe form of COVID-19 is severe respiratory failure, which requires intubation and is associated with a high mortality rate.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Pharmaceutical Development Group
AUGUST 9, 2021
Formulation chemistry is the systematic and step-by-step approach to pharmaceutical development. Nowadays, as the usage of medicines is increasing, pharmaceutical companies are more eager to bring manufacturing a new look in quality and performance. It involves designing, analyzing, and controlling manufacturing by taking timely measurements to ensure final product quality, efficacy, and safety.
Crown Bioscience
AUGUST 2, 2021
In this blog we will explore immune-mediated inflammatory diseases (IMIDs) —which represent a diverse group of conditions characterized by an excessive and/or inappropriate immune response. Interest in IMIDs has grown rapidly in recent years, largely fueled by the view that IMIDs share common inflammatory pathways, and therefore, there is the possibility that novel therapies that work in one IMID may work in other IMIDs.

Drug Discovery Today
AUGUST 16, 2021
Manchester, UK, August 11, 2021: Monument Therapeutics, a stratified medicine company, today announced Monument Therapeutics the appointment of Dr Julian Gilbert to its Board as a Non-Executive Director. Monument recently raised £2.625 million in seed funding and applies a novel drug development strategy, leveraging digital assessments of cognition to match patients with new pharmaceutical treatments.
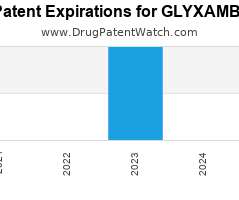
Drug Patent Watch
AUGUST 14, 2021
Annual Drug Patent Expirations for GLYXAMBI Glyxambi is a drug marketed by Boehringer Ingelheim and is included in one NDA. It is available from one supplier. There are eleven patents…. The post New patent for Boehringer Ingelheim drug GLYXAMBI appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
AUGUST 20, 2021
Today’s guest post comes from Ian Ocilka, Senior Vice President of Client Solutions at ConnectiveRx. . Ian discusses how mobile platforms like mobileCare Manager can boost patient adherence to specialty therapies. To learn more, register for ConnectiveRx's free panel discussion on September 15, 2021, at 1:00 p.m. ET. Email inquiries@ConnectiveRx.com with any questions.
The Pharma Data
AUGUST 30, 2021
Late-breaking abstract highlights a network meta-analysis on monthly migraine day reductions with AJOVY, Nurtec ® ODT (Rimegepant) and Atogepant in the preventive treatment of episodic migraine. TEL AVIV, Israel & PARSIPPANY, N.J.–(BUSINESS WIRE)– Showcasing Teva’s Commitment to Helping Patients Have More Migraine-Free Days, 18 Abstracts will be presented, Including One Late-Breaker, on AJOVY ® (fremanezumab-vfrm) Injection at the International Headache Society and European Hea

Practical Cheminformatics
AUGUST 31, 2021
A pointer to the fastpages site.

Eye on FDA
AUGUST 4, 2021
As more drugs are being approved, is FDA getting less advice than in the past? FDA maintains a vast network of outside advisors to provide input and counsel to the agency related to decisions on policy as well as product approvals. There are 31 advisory committees on matters ranging from food to medical devices to pediatric care. Within those focused on human drugs , there are 17 committees organized under therapeutic categories (Arthritis, Cardiovascular and Renal, Gastrointestinal, e.g.).
Let's personalize your content