Biogen Advances Investigational SMA Therapy to Registrational Trials After Positive Phase 1 Data
The Pharma Data
JUNE 25, 2025
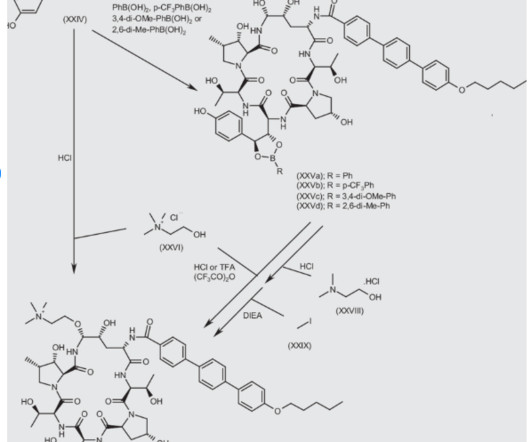
Phase 1 Study Overview The Phase 1 clinical program was structured as a single ascending dose study to assess safety, tolerability, and pharmacokinetics of salanersen. Strategic Collaboration with Ionis Pharmaceuticals Salanersen originated from Biogen’s longstanding collaboration with Ionis Pharmaceuticals, Inc. ,

































Let's personalize your content