Two HIV vaccine trials show proof of concept for pathway to broadly neutralizing antibodies
Science Daily: Pharmacology News
MAY 15, 2025
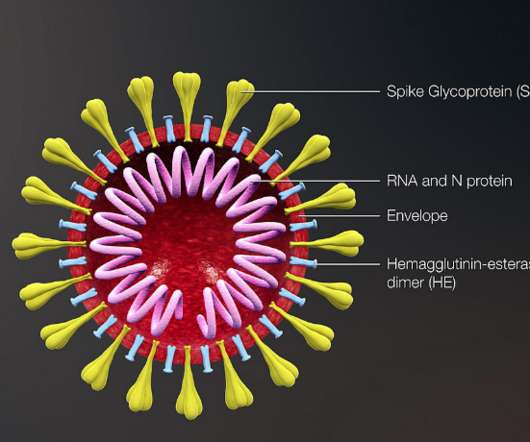
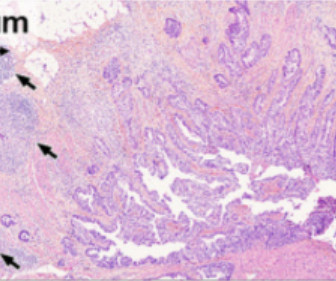
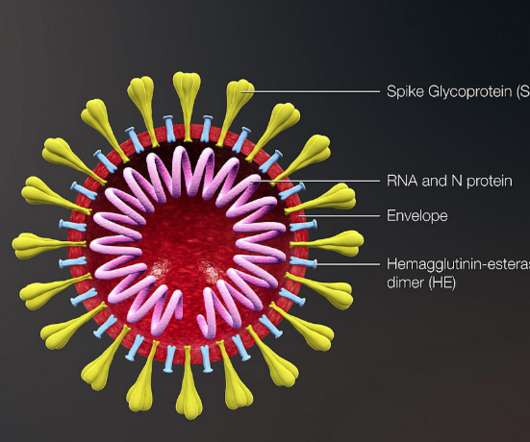
Traditional approaches haven't worked -- largely because HIV mutates rapidly and hides key parts of itself from the immune system.































Let's personalize your content