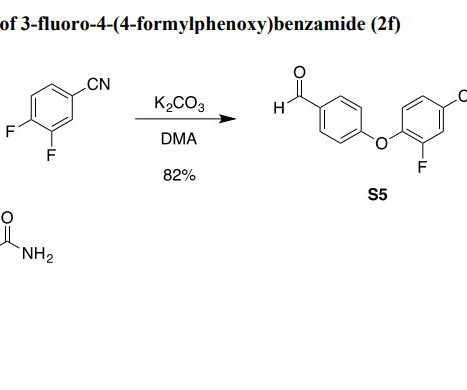
Time for change: non-human primates in drug research
Drug Target Review
JULY 7, 2025
Their unique suitability has made them valuable for evaluating pharmacokinetics, toxicology and safety in drug candidates before human clinical trials. While some therapeutic areas still rely heavily on NHPs – like biologics and gene therapies – many others are exploring models that offer better scalability and ethical acceptability.



































Let's personalize your content