Understanding the Regulatory Environment in Japan for Generic Drug Development
Drug Patent Watch
DECEMBER 30, 2024
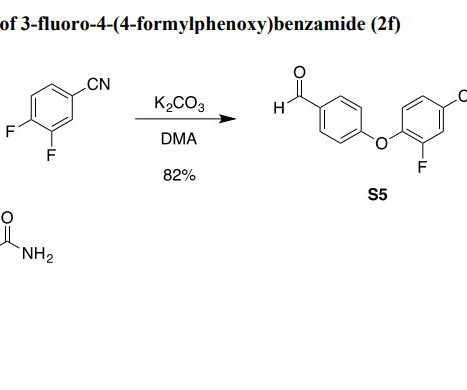
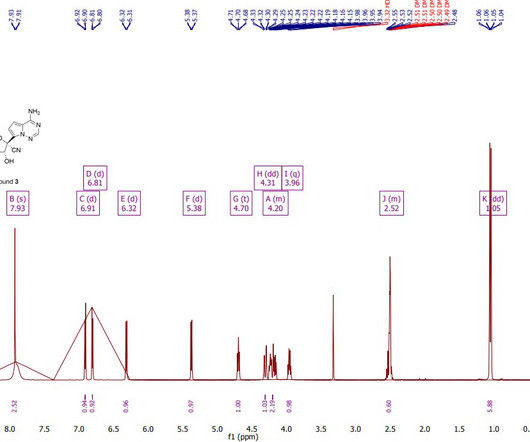
Key Requirements for Generic Drug Approval Bioequivalence Studies : The PMDA requires bioequivalence studies to ensure that the generic drug is equivalent to the RLD in terms of its pharmacokinetic and pharmacodynamic properties. References Tanaka, M., Clinical Pharmacology & Therapeutics , 111(3), 531538.









































Let's personalize your content