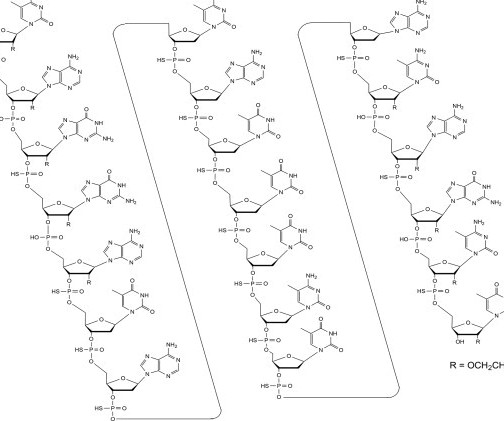
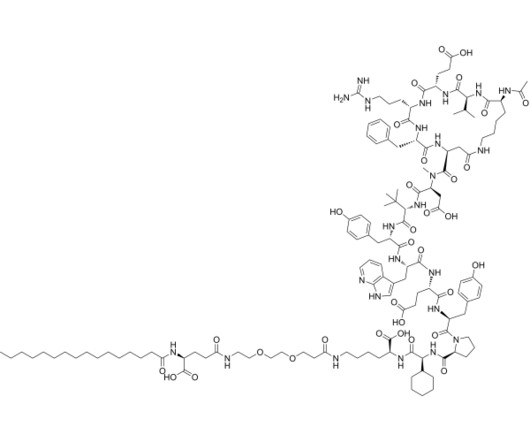
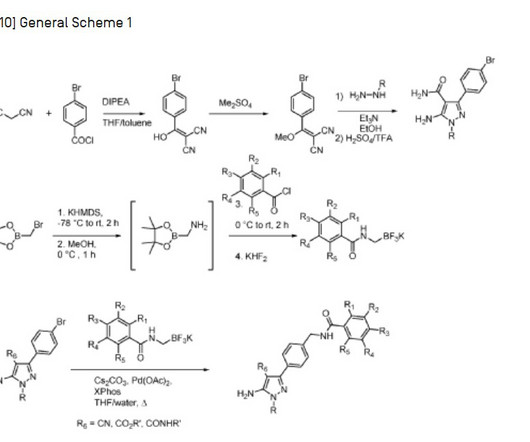
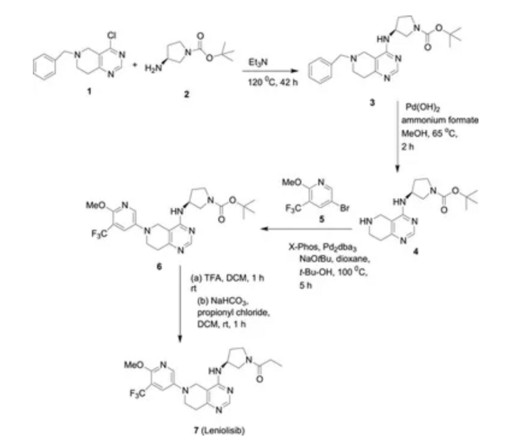
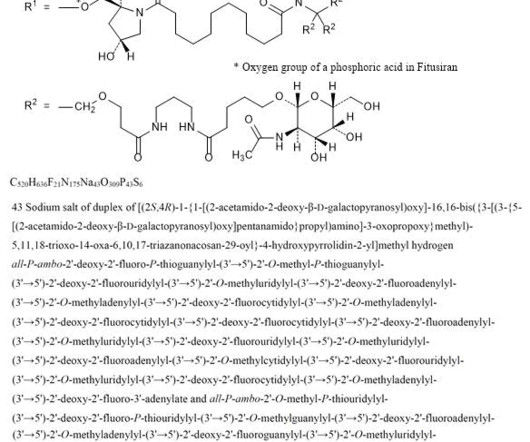
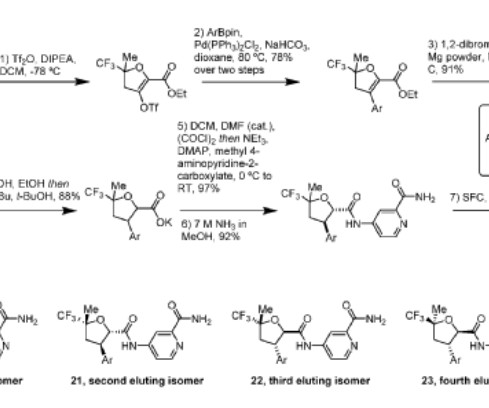
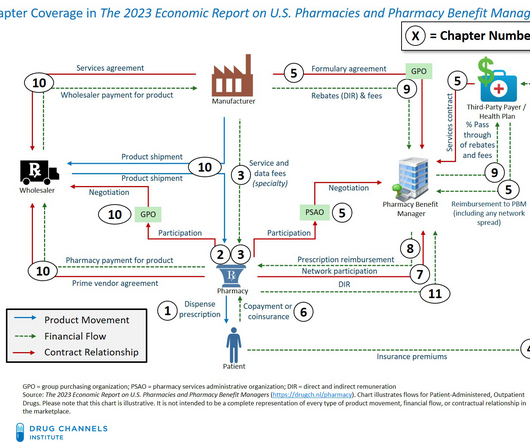
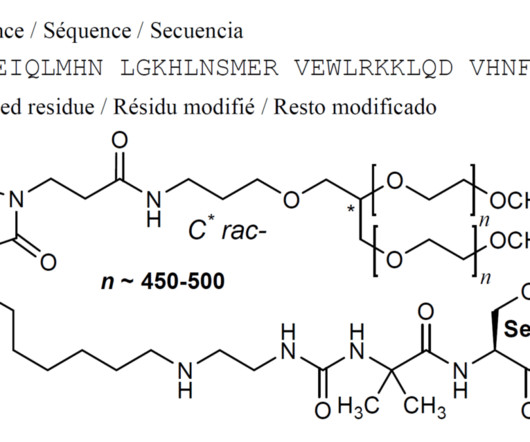
Tofersen
New Drug Approvals
MAY 17, 2025
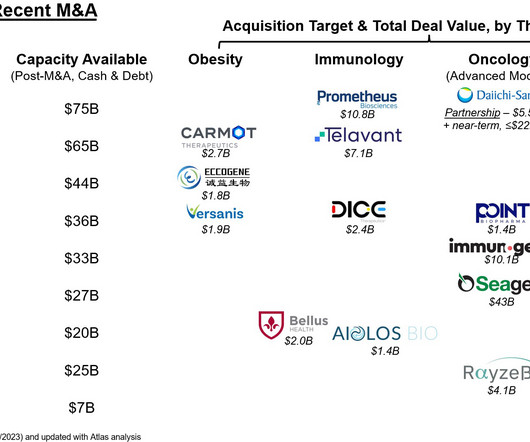
3] Tofersen was approved for medical use in the United States in April 2023, [3] [6] and in the European Union in May 2024. [4] 25 April 2023. Archived from the original on 8 May 2023. Retrieved 10 June 2023. 25 April 2023. Archived from the original on 25 April 2023. Retrieved 25 April 2023.









































Let's personalize your content