Markup Madness 2025: Hospitals, Insurers, and the Broken Buy-and-Bill Market for Biosimilars
Drug Channels
AUGUST 5, 2025
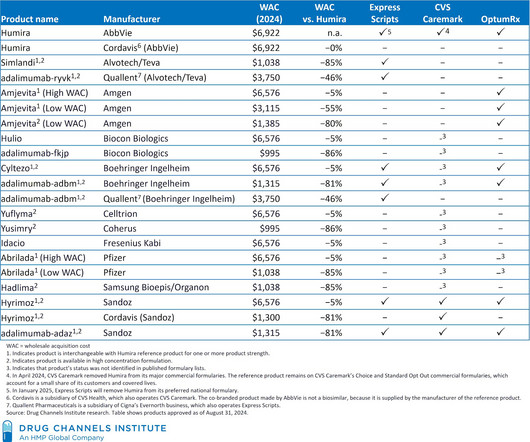
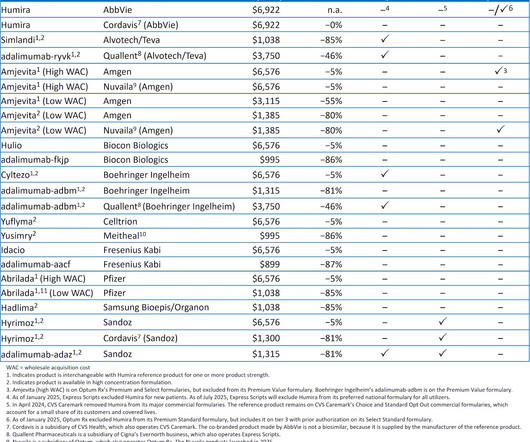
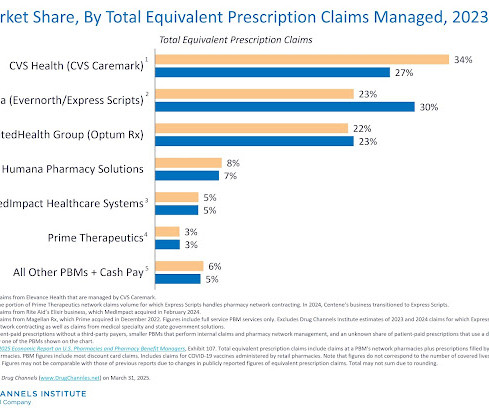
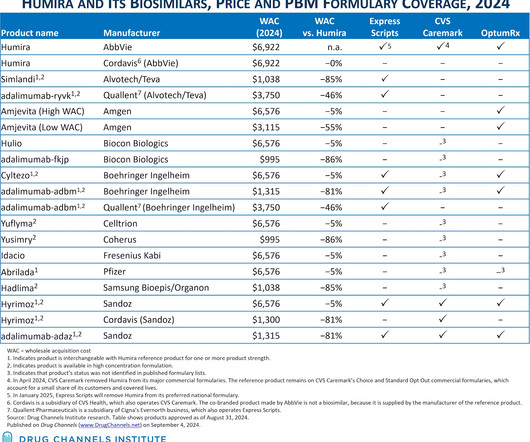
After multiple years of mandated disclosure of negotiated hospital-insurer rates, those of us who follow the buy-and-bill channel might have expected transparency to reduce drug price variability, lower hospital markups, and accelerate adoption of lower-cost biosimilars. Insurers pay wildly varying amounts for the same drug.











































Let's personalize your content