Collaboration aims to reduce animal use in drug safety testing
Broad Institute
JULY 29, 2025
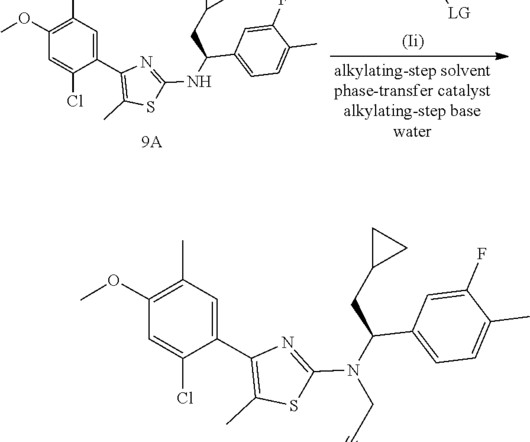
Collaboration aims to reduce animal use in drug safety testing By Leah Eisenstadt July 29, 2025 Breadcrumb Home Collaboration aims to reduce animal use in drug safety testing OASIS consortium is evaluating omics tools in cells, including Cell Painting and transcriptomics, to predict liver toxicity of potential new medicines and agrochemicals.





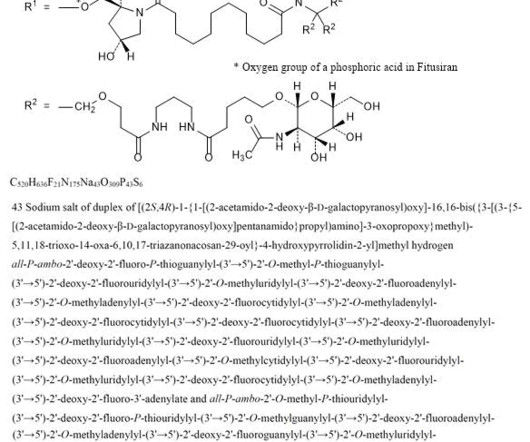



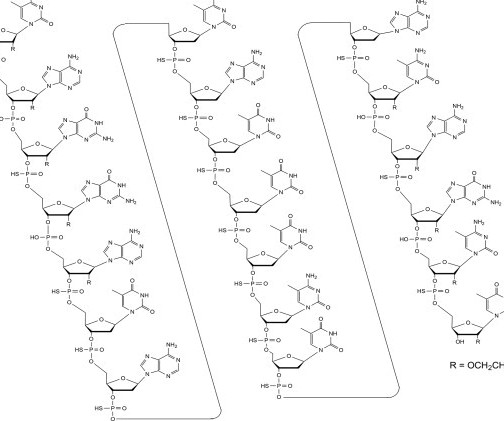

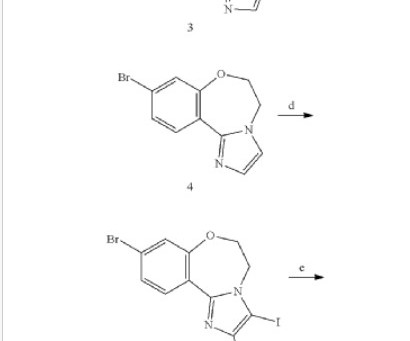






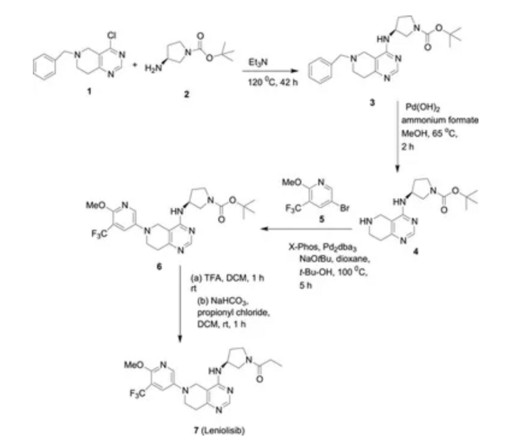























Let's personalize your content