Building Korro Bio: A CEO’s Perspective on Innovation and Risk Management
LifeSciVC
JUNE 12, 2025
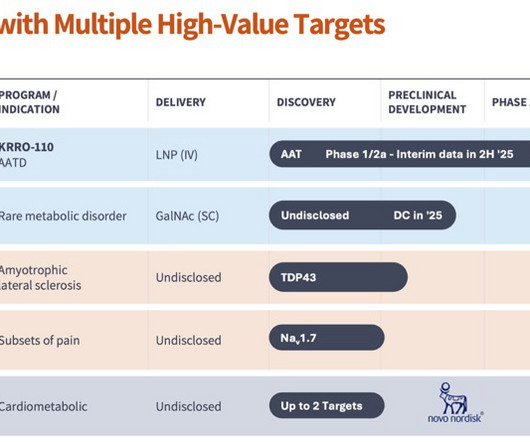
This ability to activate pathways could be possible for almost all proteins, however the biggest differentiation was to consider areas that were hard to drug – transcription factors, ion channels, intracellular proteins, etc. We intended to learn from nature (genetics) and use pharmaceuticals properties to drive patient benefit.
































Let's personalize your content