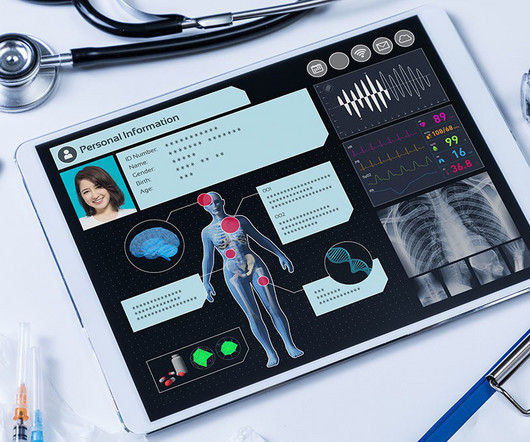
Leveraging AI Solutions for Clinical Trial Efficiencies
PPD
JANUARY 15, 2025
As a result, drug developers make better decisions more quickly (removing 50% of study timeline whitespace) to bring new therapies to market faster. Ready to learn more about how to leverage AI and machine learning to maximize your clinical trial efficiency? Get in touch.
































Let's personalize your content